DNA Tumour Virus Lab
The Macdonald group embraces interdisciplinary research and many of our projects are in collaboration with colleagues in Leeds and around the world. We firmly believe in this research model and the benefits that are acrued by sharing research ideas with colleagues.
We study four major human pathogens in the laboratory.
1. Human papillomavirus (HPV).
2. BK Polyomaviruses (BKPyV).
3. JC Polyomavirus (JCPyV).
4. Merkel Polyomavirus (MCPyV).
Members of the Papillomavirus and Polyomavirus families are the causative agents of a number of severe diseases in humans. HPV is associated with 5% of all human cancers, most notably cervical cancer in women and head and neck squamous cell carcinoma in men and women. Worryingly, HPV infection is also associated with an increased incidence of anal, vaginal and penile cancers. A number of human polyomaviruses are known to cause disease in immunosuppressed patients. In particular polyomavirus-associated nephropathy (PVAN), caused by BKPyV, is a significant cause of kidney transplant rejection and progressive multifocal leukoencephalopathy (PML), caused by JCPyV, is a life threatening demyelinating disease in a sub-set of patients receiving immunosuppressive therapies for chronic conditions including multiple sclerosis. Additionally, the Merkel cell polyomavirus is a causative agent of the highly aggressive skin cancer, Merkel cell carcinoma (MCC), which is increasing in incidence around the world. Current therapeutic strategies to treat these diseases are lacking. We have established a multi-disciplinary research group to undertake a broad ranging analysis of these viruses in the hope that a greater knowledge of their biology may yield new targets for therapeutic intervention.
1. Human papillomaviruses (HPV).
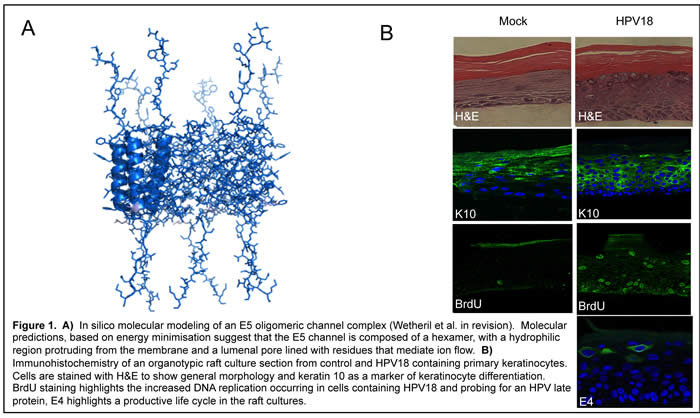
Despite the availability of a vaccine, the incidence of HPV-associated cancer is unlikely to be significantly reduced for decades and the lack of effective pre-existing anti-viral therapeutics highlights the need for a greater understanding of the mechanisms of HPV pathogenesis. We have a major research focus to study the molecular mechanisms HPV utilises to regulate an infected cell. We focus our attention on the least understood of the three transforming proteins encoded by HPV. E5 is a small, membrane protein expressed by all carcinogenic papillomaviruses. Little is known of the role of E5 in the virus life cycle or its mechanisms of pathogenesis. We have made a number of critical breakthroughs in our understanding of this protein. Most notably, we discovered that E5 functions as a virus-encoded ion channel or viroporin (Wetherill et al., 2012). We utilised de novo models of an E5 channel complex (Figure 1A) to identify small molecule inhibitors of E5 channel function and are currently using these models to reveal the functional determinants of E5 channel function in vitro. This work is funded through a three year MRC project grant and employs Dr Emma Prescott and Rajni Bhardwaj. We work closely with Dr Stephen Griffin at St-James University Hospital and Dr Richard Foster in the School of Chemistry on this project. We have also identified novel host binding partners for E5 and are using cell biological approaches to explain their contribution to the HPV life cycle (Muller et al., 2015). We are also one of the few laboratories in the UK to have established three dimensional primary cell culture models to propagate HPV in culture. These "raft cultures" faithfully recapitulate keratinocyte differentiation to allow a detailed analysis of the entire HPV life cycle (Fig 1B). We are using this system to elucidate the function of E5 in the HPV life cycle. Our data reveals that E5 may play a more substantial role in HPV replication than originally realised. These studies are a close colaboration with Dr Sally Roberts at the University of Birmingham. Our multi-faceted approach allows us to make great progress in uncovering the mysteries of E5 and we are now recognised as s a leading laboratory worldwide for the study of E5