Respiratory syncytial virus
Human respiratory syncytial virus (HRSV) is the leading cause of respiratory infections in children, causing approximately 160,000 deaths/year. In addition, HRSV infections also pose a serious risk to the elderly and immune-compromised, with significant mortality in this sector of the population. Anti-HRSV therapeutic measures are either poorly effective or prohibitively expensive, and a vaccine is not currently available. To develop improved anti-HRSV preventions and treatments, a better understanding of HRSV at the molecular level is required. We have have recently pursued several lines of HRSV research, with topics ranging from the structural biology of HRSV proteins using both X-ray crystallography and electron microscopy, through to projects more focussed on interactions between HRSV and the host cell. Information about this past work can be found by looking at the list of publications from our laboratory, which can be accessed from the faculty web page.
Current HRSV projects on-going in the laboratory:
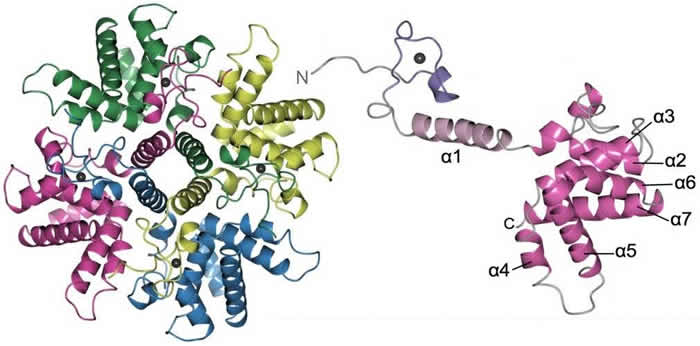
1). Structural and functional analysis of the HRSV M2-1 protein.
M2-1 is a component of the HRSV polymerase complex, and acts as a transcription factor, increasing the processivity of the polymerase during the transcription of long mRNAs. The M2-1 protein is essential for virus viability, possibly because it is required for the transcription of larger viral mRNAs, although this remains to be directly tested. The fact that M2-1 is essential for the virus life cycle makes it a good target for design of anti-viral compounds that could interfere with its function. We are focused on understanding how the M2-1 structure relates to its function, with the aim of identifying the residues that are critical for polymerase processivity and RNA binding. This project has been a long-term collaboration with Dr Thomas Edwards (University of Leeds, UK) and we recently determined the crystal structure of M2-1, with this work published in PNAS in 2014. This paper describes the native M2-1 structure, its RNA binding activities, and how phosphorylation affects its structure and function.
More recently we have been investigating how M2-1 associates with its many other binding partners including HRSV N, P, L and M proteins, as well as using rational and stochastic approaches to design inhibitors that block critical M2-1 functions. This work is funded by the MRC, and is a collaboration between Dr Thomas Edwards and Dr Richard Foster (Chemistry).
2). The role of both cellular and virus-encoded ion-channels in HRSV life cycle.
Alteration of intracellular ion concentrations is a well-established theme in disease, and one that is emerging within virology. Several viruses are known to affect the function of cellular ion channels resulting in disrupted ion balance leading to cellular dysfunction, and the list of viral proteins with the potential to affect membrane permeability is increasing. These viral proteins are collectively referred to as viroporins, and one such protein is the HRSV small hydrophobic protein (SH). We are interested in both how HRSV affects the function of cellular ion channels during an infection, and also the role of SH in the regulation of cellular ion concentration. This project is a collaboration between Dr Michael Teng (University of South Florida, Tampa, USA), Dr Peter Collins (NIAID, Bethesda, MD, USA) and Dr. Jamel Mankouri (University of Leeds), and has utilized funds from both the British Lung Foundation and the Kuwait government, in support of a PhD studentship.
More details of these projects can be obtained by directly contacting the laboratory.
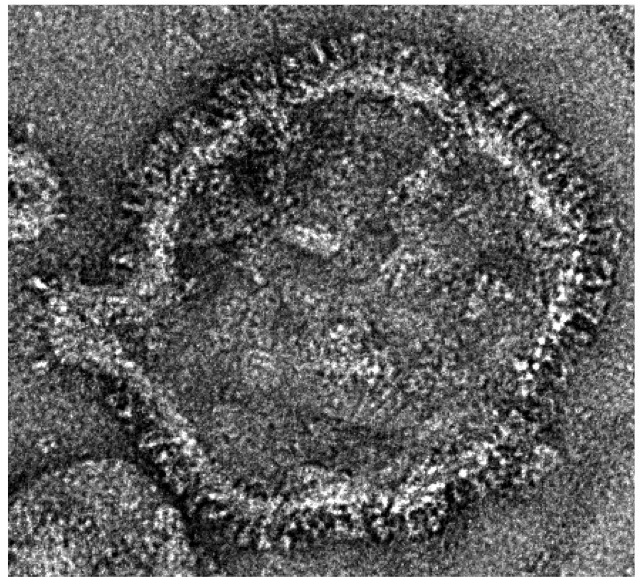
Negative stained EM image of a purified HRSV particle. Virus grown and purifed by Dr. Weining Wu, image taken by Kyle Dent.
