Introduction
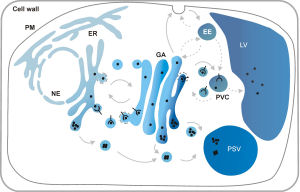 In plant cells, many proteins, lipids and carbohydrates are synthesized and transported by the secretory pathway, a complex network of organelles that mediates biosynthetic or anterograde transport starting from the endoplasmic reticulum (ER) as well as endocytic or retrograde transport from the plasma membrane (PM). The Golgi apparatus (GA) plays a central role in distributing proteins to various locations, but a growing number of differentiated post-Golgi compartments (endosomes) are thought to play a similar important function in secretion, vacuolar and endocytic transport processes. Several protein sorting signals and receptors have been identified but the challenge is now to understand how the sorting machinery functions effectively, i.e. how do sorting receptors enter transport vesicles in one compartment, but use a different transport carrier when they are recycled from another compartment. To dissect such and other basic mechanisms, it is no longer sufficient to document the consequences of defective genes, we are working with the gene products themselves using a broad range of methods including genetic engineering, protein chemistry, live subcellular bio-imaging and quantitative biochemical transport assays. Although most of our work is fundamental and curiosity driven, we also explore the knowledge gained in the production of valuable proteins in nutrition, medicine and bio-fuel production.
In plant cells, many proteins, lipids and carbohydrates are synthesized and transported by the secretory pathway, a complex network of organelles that mediates biosynthetic or anterograde transport starting from the endoplasmic reticulum (ER) as well as endocytic or retrograde transport from the plasma membrane (PM). The Golgi apparatus (GA) plays a central role in distributing proteins to various locations, but a growing number of differentiated post-Golgi compartments (endosomes) are thought to play a similar important function in secretion, vacuolar and endocytic transport processes. Several protein sorting signals and receptors have been identified but the challenge is now to understand how the sorting machinery functions effectively, i.e. how do sorting receptors enter transport vesicles in one compartment, but use a different transport carrier when they are recycled from another compartment. To dissect such and other basic mechanisms, it is no longer sufficient to document the consequences of defective genes, we are working with the gene products themselves using a broad range of methods including genetic engineering, protein chemistry, live subcellular bio-imaging and quantitative biochemical transport assays. Although most of our work is fundamental and curiosity driven, we also explore the knowledge gained in the production of valuable proteins in nutrition, medicine and bio-fuel production.
Research topics
The role of ER chaperones during plant stress responses
As part of a long term interest in ER chaperones and their function (Denecke et al., 1999 Denecke et al., 1995, Crofts et al., 1998), we have discovered a novel regulatory pathway that up-regulates chaperone synthesis independently of the unfolded protein response, which is also one of the earliest responses of plants to pathogen attack (VanDooren et al., 1999). We are currently trying to understand the mechanism by which upregulation of the ER chaperone BiP leads to an accelerated plant defense response. Furthermore, we have shown evidence for vacuolar targeting of the ER chaperone BiP (Pimpl et al., 2006) and are currently exploring if this is a property of BiP itself or if quality control mechanisms direct a specific subset of BiP-ligands to the vacuole for disposal.
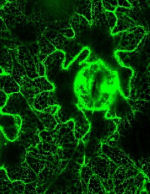 ER export
ER export
Although a lot is known about protein transport signals, little is known about the way in which sorting receptors progress through the cell. Simple fluid phase bulk flow appears to be perfectly adequate for the export of soluble proteins (Denecke et al., 1990; Vitale and Denecke, 1999; Crofts et al., 1999, Phillipson et al., 2001), but signal mediated “business class” preferred entry into transport carriers is important for transport machinery such as membrane spanning transport receptors and membrane anchored fusion mediators (SNAREs). We have shown that ER export sites (ERES) appear to be formed in a cargo signal-mediated manner (daSilva et al., 2004) and we are currently exploring ER import sites (ERIS), specific structures on the ER surface where vesicles fuse when they recycling from the Golgi apparatus. A better understanding of the two-way traffic between the ER and the Golgi is important to explain how few receptor molecules can recycle the large numbers of soluble ER residents that escape the ER via bulk flow.
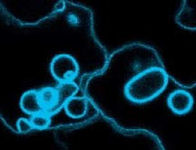
Vacuolar sorting
In plants, many of the key-players of the vacuolar transport pathways have now been identified, and plant cell biology is entering an exciting phase in which researchers want to explore how gene-products function, how receptors bind to ligands in the lumen, how ligand-binding initiates recruitment of cytosolic coats to mediate vesicle budding and how receptors recycle once the ligands are released. Using the plant vacuolar sorting receptor BP80 as a model receptor, we have mapped a variety of sorting signals in the cytosolic tail (daSilva et al., 2005, daSilva et al., 2006) and currently dissect the different post-Golgi organelles that BP80 transits when delivering its cargo and the proteins that BP80 may encounter on its way.
 Vesicle targeting and fusion machinery
Vesicle targeting and fusion machinery
A specific group of predominantly membrane anchored coiled-coil domain proteins, the so called SNAREs, act as membrane fusion mediators, a process that is crucial for vesicle fusion with target membranes as well as homotypic fusion of organelles, such as nascent vacuolar compartments. We have discovered that overdose of the prevacuolar syntaxin Pep12 reveals inhibitory SNARE activity in the vacuolar sorting route and causes secretion of vacuolar cargo (Foresti et al., 2006). Little is known about the mechanisms by which syntaxins reach their acceptor membrane, and we are currently exploring the mechanisms by which the prevacuolar syntaxin Pep12 is sorted from the vacuolar syntaxin Vam3. This research is important because SNAREs should not only target to their final destination, but specific mechanisms must ensure that they do not mediate membrane fusion whilst in transit through the secretory pathway.
 Applications
Applications
We are currently exploring the tools generated to modify protein transport in the secretory pathway to minimize proteolysis and maximize productivity. The strategies are tested in our well-established transient expression system, but also implemented in newly designed bio-fermentation units optimized for plant cell cultures. Possible products may include proteins of high nutritional or pharmacological value as well as starch hydrolases for biofuel production.
For potential PhD projects click here
Selected Publications
Foresti, O., daSilva, L.L.P. and Denecke, J. (2006) Overexpression of the syntaxin PEP12 inhibits transport from the prevacuolar compartment to the lytic vacuole in vivo. Plant Cell 18, 2275–2293.
daSilva, L.L.P., Foresti, O., and Denecke, J. (2006) Targeting of the plant vacuolar sorting receptor BP80 is dependent on multiple sorting signals in the cytosolic tail. Plant Cell 18,1477-97
Pimpl P, Taylor JP, Snowden C, Hillmer S, Robinson DG, Denecke J. (2006) Golgi-mediated vacuolar sorting of the endoplasmic reticulum chaperone BiP may play an active role in quality control within the secretory pathway. Plant Cell 18, 198-211.
da Silva L., Taylor J.P., Hadlington J.L., Hanton S.L., Snowden C.J., Fox, S.J., Foresti O., Brandizzi F., Denecke, J. (2005) Receptor salvage from the prevacuolar compartment is essential for efficient vacuolar protein targeting. Plant Cell 17, 132-148.